|
|
|
|
|
|
|
|
|
|
|
|
|
 |
Volcanic Events, pg. 2 Mount St. Helens History, pg. 3-15 Eyewitnesses, pg. 53-67 Absolute Times, pg. 81-82, 86 Activity Sequence, pg. 127-134 Gas Studies, pg. 190-191 |
Chemical
Compositions, pg.
233-250
Ash Clouds, pg. 323-333 Blast Dynamics, pg. 379-400 Rapid Deposition, pg. 466-478 Phreatic Explosions, pg. 509-511 New Lava Dome, pg. 540-544 Ash-Fall Deposits, pg. 568-584 Water Chemistries, pg. 659-664 River Water Quality, pg. 719-731 |
THE 1980 ERUPTIONS OF MOUNT ST. HELENS, WASHINGTON
PROPERTIES OF GASES AND WATERS OF DEEP ORIGIN
NEAR MOUNT ST. HELENS
By I. BARNES, D. A. JOHNSTON, W. C. EVANS, T. S. PRESSER, R. H. MARINER, and L. D. WHITE
ABSTRACT
Fluids discharging from depth
in the Mount St. Helens area include a metamorphic brine, as represented
by Pigeon Springs water discharging from metavolcanic rocks to the west,
CO2 from the mantle as represented by the CO2 well
field at Klickitat, and CO2 from the breakdown of organic matter
as represented by the gases from springs north and south of Mount St. Helens
and in a pond in the April 1980 crater.
INTRODUCTION
Carbon dioxide (CO2) is a major gas component in some explosive eruptions (Johnston, 1980; Barnes and McCoy, 1979), in some hot springs (White and others, 1973; Mariner and others, 1975; Mariner and others, 1980), and in discharges from cold volcanic sources (Johnston, 1978, p. 78) and throughout the world in seismically active areas (Irwin and Barnes, 1980; Barnes and others, 1978).
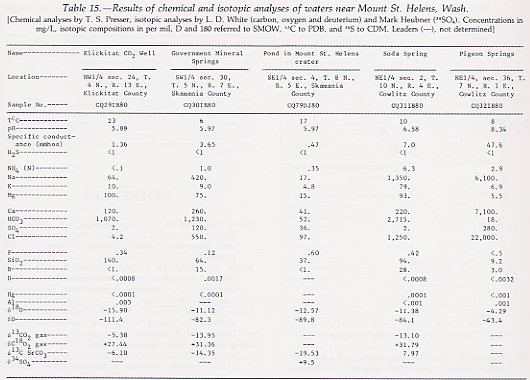
As a guide to the interpretations of the 13C compositions referred to the well-known PDB standard, 13C values in the range + 3 to -- 3 per mil are typical of marine carbonates (Craig, 1953) and values of - 20 per mil and more depleted are typical of organic matter (Craig, 1953). Mantle-derived 13C lies in the range -- 5 to -8 per mil (Pineau and others, 1976; Moore and others, 1977). Meteoric water has isotopic compositions given by the equation (Craig, 1961) ------ where D and 18O are the deuterium and oxygen compositions in per mil relative to SMOW (standard mean ocean water).
Methods used for analyses of the waters are summarized by Presser and
Barnes (1974). Gas samples were collected in evacuated glass sample tubes
with high vacuum stopcocks and were analyzed with a gas chromatograph.
Strontium carbonate samples were precipitated in the field and the CO2
subsequently evolved by treatment with H3PO4. All
bicarbonate titrations were done in the field except for the water sample
from the pre-May 18 crater, which was titrated in the laboratory. Isotope
analyses were made by L. D. White in the laboratory of J. R. O'Neil.
FIELD RELATIONS
Soda Spring, Mount St. Helens, and Government Mineral Springs lie on a line (fig. 130) that extends south-southeast to Mount Hood. Water was collected on April 23, 1980, from a pond 15 m in diameter that occupied the bottom of the summit crater of Mount St. Helens. Gas escaping through the pond raised the surface as much as 15 cm about 2 m from the shore of the pond, and there was a strong odor of hydrogen sulfide (H2S) in the crater.
The sampled well at the Klickitat CO2 field, east of Government
Mineral Springs, discharges irregularly, erupting CO2 and gas-laden
water for 3 min at half-hour intervals. Pigeon Springs is to the west of
Government Mineral Springs (fig. 130) and southwest of Mount St. Helens;
the altered volcanic country rock consists of quartz, chlorite, calcite,
and albite, as indicated by optical and X-ray study.
DISCUSSION
The isotopic compositions of the CO2-rich waters at Government Mineral Springs, Soda Spring, the Klickitat CO2 well, and water from the pond in the Mount St. Helens crater are all in the range of meteoric water (table 15 (ABOVE); fig. 131). None of them show the shift in 18O expected from water that has reacted with or exchanged isotopically with volcanic rocks (Craig, 1963). The water at Klickitat is relatively depleted in both 18O and D, because it is farther from the ocean along the path of air masses yielding precipitation.
The isotopic compositions of the CO2 being discharged show two sources. For the CO2 at Klickitat, 13C is -5.38 to -6.10 per mil, a value characteristic of CO2 from the mantle. The other CO2 discharges are more depleted in 13C, especially that from the crater pond (-19.53 per mil), probably due to decomposition of organic (woody) material incorporated in older subsurface volcanic rocks. From the pH and HCO3- values, the calculated CO2 pressure that would be in equilibrium with the dissolved CO2 is 0.05 atm, more than two orders of magnitude higher than the CO2 pressure in air.
In contrast to the CO2-rich meteoric waters at the above locations, nitrogen-rich nonmeteoric water discharges from Pigeon Springs. Neither the 18O nor the D concentrations at Pigeon Springs resemble those of local meteoric water, nor do the isotopic compositions plot on the meteoric water line (fig. 131). Pigeon Springs water resembles metamorphic waters of non-meteoric origin (White and others, 1973). In contrast to the complex chemistry of most metamorphic waters, Pigeon Springs water also resembles seawater that has reacted with rocks to lose Na and gain Ca, and its Cl concentration (22,000 mg/L) is similar to that of modern seawater (19,000 mg/L Cl). The negative D of Pigeon Springs water is at variance with this interpretation, however. All known silica-alumina hydrates are depleted in D relative to water they are in equilibrium with (Friedman and O'Neil, 1977, fig. 40), and if seawater of 0 per mil deuterium reacted to form chlorite, positive 6D values would result.
Although the origin of the saline water of Pigeon Springs is unclear, its relations to the minerals of the altered volcanic rock are known. The reaction states of Pigeon Springs water with respect to possible coexisting minerals were calculated (table 17) by computer (Kharaka and Barnes, 1973). The calculated states are from the equation where GR is the free-energy difference between the equilibrium state and the actual state, and R is the gas constant, T is the temperature (absolute), Q is the reaction quotient, and K is the equilibrium constant.
The states of reaction and the mineral composition of the altered volcanic rock at Pigeon Springs are compatible. The water is supersaturated with respect to the quartz, chlorite, albite, and calcite that constitute the altered volcanic rock and thus could be the metamorphic fluid existing when the present minerals of the rock formed. Although the water is supersaturated with respect to many clays, they cannot precipitate because the water is supersaturated with respect to the minerals now in the rock, and hence a free-energy barrier exists to clay-forming reactions. Thus, the minerals found and the water described can coexist indefinitely with no further reaction possible. If, however, the brine is removed and fresh (meteoric) water enters the rock, the calcite-low albite pair will be incompatible and will react to form laumontite (Barnes and others, 1978).
THE 1980 ERUPTIONS OF MOUNT ST. HELENS, WASHINGTON
FUMAROLE ENCRUSTATIONS:
OCCURRENCE, MINERALOGY, AND CHEMISTRY
By TERRY E. C. KEITH, THOMAS J. CASADEVALL, and DAVID A. JOHNSTON
ABSTRACT
Fumaroles associated with
the 1980 eruptive activity of Mount St. Helens occur (1) within the crater,
where heat and fluids may be derived directly from magma and from crustal
rocks in contact with the rising magma, and (2) as rootless fumaroles in
the flowage deposits, where heat and fluids are derived locally from within
the deposits. Much of the encrustation material was deposited at temperatures
less than 250°C as noncrystalline yellowish films on ash particles
and rock fragments and as encrustations around fumarole vents. Encrustations
are mostly red, orange, yellow, and white. They begin to crystallize during
cooling and dehydration immediately after deposition. Common mineral phases
are sulfur, gypsum, halotrichite, and hematite. Less common phases are
sal ammoniac, thenardite, glauberite, anhydrite, melanterite, alunite,
and halite. Numerous unstable and poorly crystalline phases in process
of dehydration and crystallization are under study. The major chemical
components making up the fumarole deposits are Cl, F, H2O, SO4,
Fe, Al, Ca, Na, K, and S.
INTRODUCTION
A fumarole is a vent that emits only gases and vapor. Encrustations are the colorful yellow, orange, red, and white deposits that generally surround fumaroles. Sublimate, a term often used for these encrustations, refers specifically to a solid phase deposited directly from a gaseous state without going through a liquid phase; the term is applicable to most encrustations, but not all.
Our study of fumaroles at Mount St. Helens focuses on the occurrence
and distribution of different kinds of fumaroles and the chemistry and
mineralogy of the encrustations. Reactions and products of hydrothermal
alteration associated with the 1980 activity (Dethier and others, this
volume) are not considered here.
ACKNOWLEDGMENTS
Field studies were by T. E. C. Keith and T. J. Casadevall, and by D.
A. Johnston, who collected the first deposits from the summit crater on
April 23 and analyzed the leachates. Identifications of mineral phases
and chemical analyses were made by T. E. C. Keith, N. L. Nehring, T. S.
Presser, D. P. Dethier, and D. R. Pevear.
METHODS
Temperatures of fumaroles were measured at the hottest accessible place
in each vent. Gas and encrustation samples were collected simultaneously
at several sites (see Casadevall and Greenland, this volume). The encrustation
samples were sealed in plastic bags in the field until ready for laboratory
study by X-ray diffraction (XRD) and scanning electron microscope (SEM).
An energy dispersive X-ray analyzing system (EDAX) was used on the SEM
to determine qualitatively most of the chemical components (most cations,
Cl, and S) of the encrustation phases. Some soluble phases may have dissolved
in water which condensed in the plastic bags. The hy-dration states of
aluminum chloride, aluminum sulfate (± iron), calcium sulfate, and
iron chloride probably changed somewhat during study. The color of some
encrustations has changed from pale green (ferrous iron), to yellow, to
orange, and finally to pale brownish red, indicating that iron in these
hydrated phases oxidized after collection. Not all crystalline phases have
yet been identified; however, the common morphologies and mineral phases
have been determined by SEM and XRD.
FUMAROLES AT MOUNT ST. HELENS
Fumaroles at Mount St. Helens occur (1) within the crater, (2) on flowage deposits of the May 18 eruption and on more recent pyroclastic deposits, and (3) on the May 18 blast deposit (fig. 132). The fumaroles within the crater may be connected directly with a magmatic source of gas and heat. Fumaroles on the flowage deposits and on the blast deposit are termed "rootless" because they are not connected directly to a magmatic source of heat or gas.
Prior to 1980, Mount St. Helens exhibited little thermal activity. Phillips
(1941) reported weak fumarolic activity (T = 88°C) at The Boot, at
2,740 m elevation on the north flank. A second thermal area at approximately
2,740 m elevation on the southwestern flank of the volcano (T = 89°C)
was reported by Friedman and Frank (1977). Both thermal areas were destroyed
on May 18.
FUMAROLES WITHIN THE CRATER
Within the crater, fumaroles occur (1) on the surface and at margins of successive dacite domes, (2) in cracks of the tephra blanket adjacent to the dome (fig. 133), and (3) along the intersection of the crater wall and the floor. Types 1 and 3 were not accessible during our sampling. Fumaroles of type 2 were studied on September 16 and 25 during visits into the crater.
The first dome, initially observed on June 15, was partially destroyed on July 22 (Moore and others, this volume). A second dome appeared in the crater following the eruption of August 7; a tephra blanket surrounded this dome and covered remnants of the June dome. Cracks in the tephra blanket, first observed on September 8, radiated northeast, north, and northwest from the dome. The cracks were as much as 50 cm wide, and many opened appreciably between visits on September 16 and 25. Incandescent rock, probably of the June dome, was locally visible in the cracks, and temperatures in them ranged from less than 200° to 838° C. Gases responsible for the deposition of encrustations were also probably derived from the June dome.
In the hottest (northeast) fracture, the crack walls contained no encrustation coatings but had apparently been slightly oxidized to a reddish, friable rubble. Yellow encrustation material was abundant on the surface a few centimeters outward from the cracks (fig. 133). This material is noncrystalline and is composed of Al, Cl, Fe, Ca, S, and H2O. Dehydration cracks developed (fig. 134), and blades of a crystalline material (Cl, Al, Fe, S, Ca, + water) began to form (fig. 135), finally resulting in a fuzzy bladed coating (fig. 136). Native sulfur was rarely observed as crystals on the ground surface adjacent to major cracks.
Deposition of a sublimate film from fumaroles within the crater began at temperatures below about 250°C. Rapid cooling of the almost colorless vapor produced a pale-yellowish-brown stain on crack walls. The stain did not obscure the textures of ash or rubble in the crack (fig. 133A). This stain material is soluble in water, has a high Cl-1 content (N. L. Nehring, written commun., 1980), and appreciable Fe+3, F-1, SO4-2, and Al (T. S. Presser, written commun., 1980).
Volcanic ash collected by David Johnston from the summit crater on April
23, 1980, was coated with similar yellowish film. Field analysis of the
leachate of this film material showed appreciable Cl-1 and SO4-2.
SEM study showed only ash particles, but EDAX analyses of the particle
surfaces showed high Fe and Cl content, which must have been in some material
forming very thin coatings on the ash particles. Scarce gypsum crystals
had formed on the surface of one rock associated with the yellow deposit.
ROOTLESS FUMAROLES
Locations of rootless fumaroles on the flowage deposits were controlled by features such as preemption surface drainage, contrasting permeability of flowage deposits, and posteruption fractures produced by cooling or by settling of flowage deposits. Most rootless fumaroles that were active more than a few days were associated with pyroclastic flows that extended from north of the crater to Spirit Lake or with the debris-avalanche deposits in the North Fork Toutle River east of the mouth of Coldwater Creek (fig. 132).
Organic material (tree trunks, branches, and needles) is common in the
flowage deposits of May 18. Where the deposits were hot, this material
was distilled to varied products with sweet, turpentinelike odors. These
distillates mostly escaped from individual small fumaroles or rows of fumaroles,
and the ash overlying the organic material was discolored or coated by
brownish-red material. When temperatures were high enough, the trees
smoldered or burned. As temperatures decreased, fumaroles over buried trees
became fertile places for growth of fungi, algae, and, eventually, new
plants.
PYROCLASTIC ROWS
Each pyroclastic flow had associated fumarolic activity. Temperatures were as high as ~850°C in these flows (Banks and Hoblitt, this volume), but fumarolic encrustations do not appear to have formed until the near-surface zone (less than 1-m depth) cooled to about 250°C or less. Of the pyroclastic flows examined (5/18, 5/25, 6/12, 7/22, 8/7), only the flow of June 12 contained solfataric fumaroles (fig. 137), distributed evenly over its surface at roughly 1- to 1.5-m spacing. These solfataras developed within a day of emplacement of the flow and persisted for several weeks before sulfur deposition declined. Gases from these fumaroles consist mostly of air and steam (Casadevall and Greenland, this volume).
Encrustations at fumaroles on pyroclastic flows are typically crusted with nearly white deposits, tinted with reddish iron oxides or hydroxides. Most contain an assemblage of gypsum and (or) anhydrite, iron oxide (hematite) or iron hydroxide, and native sulfur. Low-temperature fumaroles on the pyroclastic flows are yellow, as they are coated with native sulfur (fig. 137).
Iron leached from mafic material in dacitic ash is precipitated as an
orange to red coating on the material through which the vapor passes. The
red iron-rich coatings are deposited at high temperatures, usually during
the initial stages of fumarolic activity (fig. 138). Metallic trace elements
are also present in the dark-red coating from sample MSH 5-29-80-14 (T
= 141°C; table 18). At high temperatures this reddish material is hematite;
at lower temperatures it is amorphous iron hydroxide (fig. 139). Yellow
encrustations (table 18) deposited over the dark-red material contain much
lower concentrations of metallic trace elements, especially Ag, B, Ba,
Cu, Zn, Zr, and Ga. Fumaroles 11 and 12, near the west edge of the May
18 pyroclastic flow, also exhibit this type of deposit. Anhydrite needles
partly coated with anhedral blobs of alunite (probably natroalunite, fig.
140) form a later white deposit on iron oxide associated with fumarole
12 (now buried).
BLAST DEPOSIT
Rootless fumaroles in the blast deposit generally had temperatures below
150° C and were most common within a few kilometers of the crater,
where the blast deposit is thickest. Feeble steaming at such fumaroles
generally lasted only a few days to a few weeks, probably because the blast
deposit was thin and cooled rapidly.
DEBRIS AVALANCHE
The debris avalanche is composed of material from the pre-May 18 edifice of Mount St. Helens mixed with sparse gray dacite from the 1980 cryptodome (Voight and others, this volume). These blocks of gray dacite showed little alteration or vesiculation, suggesting that they were not the major source of the volatiles responsible for the encrustations around the rootless fumaroles on the debris avalanche. Fumaroles on the debris avalanche had maximum measured temperatures of 125° to 150°C and cooled with time (fig. 141).
Fumarole 6, from the debris avalanche (fig. 141), was sampled on July
1, 1980, when the maximum temperature was 94° C. The encrustations
consisted of an orange to yellow-orange deposit in the hottest area, grading
outward to a greenish colloform deposit, surrounded by a zone of abundant
white, gelatinous colloform material. A dark-gray outer zone resulted simply
from water wetting the light-gray dacite ash. When the same fumarole was
resampled on September 16, 1980, the maximum temperature was 69°C,
and almost all orange and yellow color was gone (fig. 141B). The configuration
of the fumarole was the same, however, and the encrustations consisted
almost entirely of white colloform gypsum. The outer limit was still dark
gray because of moisture. The yellow to orange material is mostly poorly
crystalline and consists of S and Al with or without Cl, Fe, and Ca (fig.
142). As dehydration cracks develop in the material, hydrous calcium sulfate
(containing much more water than gypsum) crystallizes (fig. 143). If dehydration
continues, the stable product seems to be gypsum (fig. 144). Other phases
that ultimately crystallize as the system cools are halotrichite (fig.
145) and hydrated aluminum sulfate. Native sulfur crystallizes on some
deposits of this type as cooling continues, but little sulfur is present
in fumarole deposits on the debris avalanche. The yellowish deposits consist
largely of gypsum covered with a thin yellow coating, which seems to be
non-crystalline and composed of iron and chloride with or without sulfate.
DISCUSSION
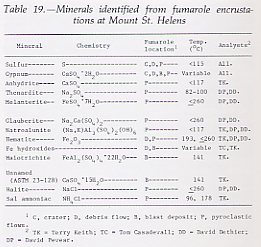
Fumaroles at Mount St. Helens apparently evolve in a predictable chemical and mineralogical pattern. For fumaroles with temperatures below about 250°C, most encrustations are white to yellowish orange and are first deposited as thin, noncrystalline deposits on rock fragments. Continued deposition results in a deposit of amorphous, often gelatinous material, typically colloform in appearance. With decreasing temperature and time, these deposits begin to crystallize and dehydrate through a series of unstable phases. Some phases are water soluble and easily leached by rain or surface drainage. The most stable phases that remain are gypsum, anhydrite, sulfur, hematite, and iron hydroxides. All minerals identified from Mount St. Helens fumaroles to date (table 19; BELOW) have been found previously as fumarole encrustations. (See Stoiber and Rose, 1974, for an excellent review.) The principal anions identified are Cl-1 and SO4-2, and the principal cations are Fe+2, Fe+3, Al+3, Na+, Ca+2, and K+; minor constituents are not yet known. Sulfur is the only element found in the native state. Nearly all phases are hydrous, but the extent of hydration is still under study.
 |
Most encrustations are deposited from fumaroles with temperatures below 250°C. In general, fumaroles on the debris avalanche were initially cooler (less than 100°C) than those on the pyroclastic flows and in the crater. This difference in temperature is reflected in the mineralogy of the encrustations: those from the pyroclastic flows tend to have hematite; native sulfur and gypsum occur in both types of fumaroles; the higher temperature encrustations typically contain abundant chlorides and sulfates of Ca, Fe, and Al; and the hottest fumaroles adjacent to the dome entirely lack encrustations. These data support the observations of Stoiber and Rose (1974, p. 513), who found that encrustations deposited at high temperatures are generally less abundant than those deposited at lower temperatures. |
Most fumarolic encrustations at Mount St. Helens are yellow to orangish yellow. Commonly, these are described by field observers as sulfur, but the color is generally due to hydrated chlorides and sulfates of Fe and Al. Only rarely did sulfur occur with a mineral other than gypsum. For example, fumarole 7, in the debris avalanche, had cooled to the point where it no longer deposited chloride-bearing minerals, and sulfur was apparently deposited over the earlier chloride assemblage. In the hotter fumaroles in the crater area and on the pyroclastic flows, encrustations are commonly buff to white with patches of iron-rich reddish-brown material.
Studies of burning refuse piles at coal mines demonstrate that mineral assemblages reminiscent of volcanic sublimates can be produced by heating finegrained sedimentary rocks (Dunrud and Osterwald, 1980; Finkleman, 1978). Many elements (alkaline earths, halides, sulfur, metals) are easily leached from fine-grained sediments and volcanic rocks by hydrothermal solutions with neutral or slightly acidic pH (Ellis and Mahon, 1967). Thus, many requisite components of fumarolic encrustations on the debris-avalanche deposits may be derived by heating the older rock of Mount St. Helens in a water-rich environment. Much rock was already heated and partly altered in the pre-May 18 hydrothermal system, and water from streams, marshes, and lakes could become an effective hydrothermal fluid capable of leaching cations and anions from rocks of the debris avalanche. Fumarolic encrustations could form following cooling of this fluid upon reaching the ground surface.
White and Waring (1963) pointed out the problems of determining which
components of fumarolic encrustations were precipitated from vapor and
which were derived from acid attack on surrounding rock particles. Our
studies are continuing on this problem.
| START & IMAGES |
|